Altace
By V. Josh. Eastern Washington University.
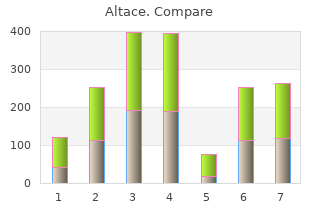
Some- times the supply falters because of shortages in the raw materials generic 2.5mg altace with amex heart attack jim jones, as in 2004 when increased demand for artemisinin purchase altace 5mg on-line blood pressure 0f 165, combined with a poor Artemesia annua harvest, drove up the price and led to stock-outs (Kindermans et al. In the United States, for example, manufacturers sometimes stop producing products with low proft margins, such as sterile injectables—inexpensive products that are complicated to make (Hoffman, 2012). Manufacturers also can lose interest in a drug after its patent expires, when revenues from the product drop (Hoffman, 2012). Although the United States has a more stable drug supply than most devel- oping countries, there have been regular shortages for the past 15 years, es- pecially among injectables, cancer drugs, and antibiotics (Hoffman, 2012). Figure 4-3 shows that although private-sector outlets have a higher percentage of drugs available than public-sector ones, there is still a great deal of unmet need. A month’s course of the lowest-priced generic ulcer medication, for example, is still more than 3 days’ wages for a low-paid government worker in much of Africa, Eastern Europe, and the Middle East (Cameron et al. Reducing the costs and increasing the availability of medicines would remove some of the fnancial incentive to produce and procure falsifed and substandard medicines. If patients had a plentiful supply of reliable, affordable medicines, there would be less need to shop at unregulated gray markets. For generic manufacturers, companies that generally run on low margins, the costs of proving bioequivalence and preparing a manufacturer’s dossier for regula- tory review can be prohibitive to market entry (Lionberger, 2008). Different regulatory authorities have different, often widely divergent, requirements for establishing bioequivalence (Mastan et al. Countering the Problem of Falsified and Substandard Drugs 164 Copyright © National Academy of Sciences. The committee believes this format could be useful to regulators and gener- ics companies in low- and middle-income countries. Harmonized applications also give regulators a common format to discuss their product registration process. Like sharing inspections and other harmonization efforts, the use of the common document increases effciency and promotes a common language among regulators. Recommendation 4-3: Regulatory authorities in low- and middle- income countries should use the International Conference on Harmoni- sation Common Technical Document format for product registration to better harmonize their procedures and reduce application costs for manufacturers. To the same end, they should also conduct joint inspec- tions and use a common inspection report. A more robust generic drug market in low- and middle-income coun- tries could help prevent the drug shortages and price spikes that encourage the sale of poor-quality products. Regulatory authorities can work to better harmonize their procedures, thereby improving their own effciency and reducing barriers to market entry for good-quality generics manufacturers. Regulators also reap a spillover beneft of more convergent regulatory systems without negotiating cumbersome mutual recognition agreements. The Singaporean drugs regulatory authority has promoted the common format, citing its ease of use and the way it facilitates sharing information among other regula- tors in the region (Poh, 2011). Similarly, Southeast Asian companies beneft Copyright © National Academy of Sciences. Bioequivalence testing also requires sophisticated laborato- ries that are not available in many countries. This baseline cost to generic companies does not include several person-months of staff costs for revis- ing registration application data into a new dossier. The costs of market authorization are prohibitively expensive, especially for entry into a small country’s market. When the overwhelmed regulatory authority will allow it, companies avoid the expense by submitting no proof of bioavailability; others falsify bioavailability data (Silverman, 2011). Evidence suggests that these high costs keep generics companies out of the market and increase costs to the consumer (Mastan et al. A 1996 industry study estimated that converting applications took between 2 and 10 months and signifcant staff time and expense (Molzon, 2009). Differ- ent standards for bioequivalence assessment also encourage the problem of widely divergent national drug quality standards (Mastan et al. If the application and registration process were more straightforward then more good-faith companies could enter the market, increasing the sup- ply of reliable drugs and controlling costs. The committee also believes that a consistent use of the common registration format could further the cause of regulatory harmonization, which would improve the drug regulatory systems in low- and middle-income countries. Harmonization also controls the burdens regulation puts on manufacturers; shared inspections are more effcient and less disruptive to industry. Generics companies, which gener- ally have fewer staff than innovator companies, are disproportionately disturbed by frequent inspections.
Reentrant tachyarrhythmias characteristically have an abrupt onset and ter- mination and a nonvarying rate during the tachycardia 10 mg altace overnight delivery blood pressure up during pregnancy. Cardiovascular Physiology Care of the patient with hemodynamic derangements remains rooted in basic physiological concepts—preload order altace 10 mg overnight delivery blood pressure 4 year old child, contractility, and afterload—first described in the late 19th century. These factors directly impact stroke volume, which, along with heart rate, are the key determinants of cardiac output (Figure 1-4). Preload, contractility, and afterload each impact cardiac output via their effects on stroke volume. Munoz Preload Preload refers to the ventricle’s intrinsic ability, within a physiological range, to alter the force of contraction based on the degree of ventricular filling just before contraction (end-diastolic volume/fiber length). The greater the end-diastolic volume, and, thus, ventricular myofiber stretch, the greater the force of contraction. Conceptually, preload is most often equated with the intravascular volume status of a patient. Contractility As already noted, within physiological range, the greater the myofiber stretch (preload), the greater the force of contraction. However, contractility (or inotropic state) specifically refers to the magnitude of response to a given preload and can be thought of as the “multiplication factor” for any given preload (Figure 1-5). Contractility is an intrinsic property of the muscle fiber that is relatively inde- pendent of changes in preload or afterload. In other words, for any given preload, the force of contraction will be greater under conditions of increased inotropy (e. Each of these therapies has multiple effects, aside from enhanced inotropy, which may limit their therapeutic efficacy (e. Afterload Afterload is defined as the ventricular wall stress during contraction and is often conceptualized as the load against which the ventricle contracts. Cardiac Physiology Review 7 increased B contractility A normal decreased contractility Left ventricular end-diastolic volume (preload) Figure 1-5. Frank-Starling curve illustrating the relationship between various preloads inotropic states and cardiac output. However, for a given preload A or B, cardiac output is, in part, determined by the inotropic state (contractility). In other words, afterload determines the size of the ventricular cavity at the end of contraction, independent of the ventricular volume before contraction (preload). Munoz Pressure-Volume Loops Visual representations of these physiological concepts can be helpful to best appreciate their individual characteristics and their impact on one another in vivo. One particularly useful way to appreciate the contributions of and interactions between preload, contractility, and afterload is by examination of pressure-volume loops. As shown in Figure 1-6a, ventricular diastolic perform- ance (compliance) and changes in preload are illustrated by the curve at the bottom of the graph (end-diastolic pressure volume relationship), ventricular volume throughout the cardiac cycle is illustrated by the rectangle, and con- tractility is illustrated by the diagonal line (end-systolic pressure volume rela- tionship). With the onset of systole (Point A), there is an increase in pressure (isovolumic contraction) until ventricular pressure exceeds aortic pressure, at which point, the aortic valve opens and blood is ejected from the ventricle (Point B). As the ventricle continues to empty, there is the onset of relaxation of the ventricle, with an eventual drop in pressure below that of the aorta (Point C). At this point, ventricular pressure falls but the volume remains unchanged (isovolumic relaxation) until the pressure drops below that of the left atrium and the mitral valve opens (Point D). The ventricular volume then increases during diastole, until the cycle repeats itself with the next contraction. The area within the rectangle represents stroke work, and the distance along the x axis between the vertical lines is the stroke volume. As illustrated in Figure 1-6b, increased preload results in a greater stroke volume as compared with baseline, but the end-systolic volume in both instances is limited by the afterload (and contractility). With decreased afterload (dash-dot line), a lesser end-systolic volume and a greater stroke volume are achieved. As shown in Figure 1-6c, alterations in contractility (inotropic state) also affect changes in stroke volume. Finally, differences in ventricular compliance (slope and shape of curve at bottom of graphs) result in differences in end-diastolic volume (myofiber stretch) for a given preload (Figure 1-6d), and, thus, also impact stroke volume. Clinical Measures of Cardiac Function and Contractility Bedside assessment and care of patients is driven, in part, by the technology available for clinical assessment. For example, although the use of impedance catheters to ascertain pressure-volume loops might best inform clinicians regarding the changing cardiovascular status of their patients (and the response to therapies), this technology is impractical in most cases because it requires an invasive procedure for placement and impractical levels of continuous monitoring. Thus, most clinicians rely on surrogate measures and their clini- cal experience to manage patients.
By this I mean real modern slavery discount 10mg altace overnight delivery prehypertension 39 weeks pregnant, where people get in trap trying to find better life buy generic altace 10mg online blood pressure log template. Those people have to work hard, it‘s forbidden for them to leave their place of work and living by their so-called ―employers‖. Moreover, ―slaves‖ can‘t communicate with their relatives or seek help from government. And the worst pert is that you can‘t break this system from inside, so it‘s in our interests to prevent these crimes against human rights. From moral point we can‘t say that it‘s not our problem, because it can happen to anyone: you, your family or friends. I believe that the main reason of modern slavery is language ―border‖, because there is no point to cry for help if no one will understand you. Today government try to integrate English into our lives, but it seems that none of it‘s methods work. Eventually this brings us to another statistical result, according to the English Language Resources less then 4 percents of people can speak English in Ukraine. Just because riding a bike seems really cool in childhood, and it‘s known that children learn everything much better and faster than adults. You might think that we have already English schools for our beloved kids, that we even have English classes in pre-school. The problem is that in schools teachers always say that the main subjects are math and Russian or Ukrainian languages. Even if you want your kid to learn English in English schools, you‘ll have to spend a lot of money. By just accepting this fact as life we are guilty for making English some kind of luxury! By achieving this we‘ll get a country that has 3 national languages, imagine our culture in 100 years after this victory. A country of culture, business and all kinds of opportunities, a country of diversity and peace. Finally to sum up all my words into single thought I want to say that difference is not makes us hate to each other, but misunderstanding is. We start conflicts with one word that was given us by God to find comprehension in each other. It all seems such a childish mistake, because we all want to help one another, that‘s our nature. This world has room for everyone and our Earth is rich, it can provide it‘s treasures for everyone. Without this qualities life will be violent and all of us will lose everything we love. The given work deals with the description of innovations in the lexical sphere in the Russian language of the modern period. The appearance of such innovations is caused by the essential changes of socio-political and economic character. Logos word) - a new word, linguistic innovation (figure of speech), the grammatical feature, which appears in the language. The increased interest to the neology problem is due to the important role of neologisms as a mirror of language development, which reflects the language adaptation to the changing under the influence of external factors, the conditions of its operation. The starting point is the practice of lexical innovation, because cultural and historical, social and political conditions of life and work of the speech community affect the lexical and nominative activity. The material of the research are neologisms, which are often found in the speech of young people - Russian native speakers and the material of student of the blogosphere. The study is a description of the material, based on the study and synthesis of the major achievements of modern linguistics and lexicography theory, their basic concepts. The main methods are: the method of observation, description and method of the survey informants - native speakers. Along with the aging process of certain words much more intensively flows replenishment process of the lexical composition of the language.
Candida is a fungus that aids with nutrients absorption ad digestion when in proper levels in the body discount altace 5 mg amex blood pressure quiz pdf. By far the most common causes of invasive fungal infections are members of the genus Candida buy 2.5 mg altace blood pressure medication manufacturers. Candida Albicans is one of the more common fungal pathogens that can colonise skin and mucous membrane all over the body and while normally harmless, can cause candidiasis or thrush infections like vaginitis. If the fungus enters into the bloodstream and streads around the body, it becomes life threatening in some cases. There are many other types of fungal infections which can be just as harmful and are also increasing in prevalence globally. The causes of Candida infections can include; birth control pills, oral corticosteroids, cancer treatments, diabetes, weakened immune system, broad spectrum antibiotics. The symptoms can include; chronic fatigue, sinus infections, mood disorder, intestinal distress, recurring vaginal and urinary tract infections, oral thrush, brein fog skin and nail fungal infections, hormonal imbalance. There have been different vaccines strategies for active immmunisation and two have gone through phase 1 of clinicaltrials against C. Albicans with an adjuvant chemical the helps stimulate the required immune response. This allows production of antibodies against the fungal antigens in the vaccine and induces protection against the infection. Active vaccination ai not ideal for immune suppressed patients as their immune system is not strong enough to produce the antibody response seen in healthier individuals. Another passive form of immunisation would need to be developed for these individuals. A live attenuated vaccine called rAls3p-N has been deeloped and was recently tested in humans after positive findings inmice and primates. In human trials, 30 subjects were administered 2 increasing doses of vaccine and no side effects were reported. The increased immune response suggests this vaccine proides 98 protection against C. Albicans has also gone through Phase 1 trials and shows tolerability and efficacy in humans. One problem with using mice is that they have a very different immune response to candida. Unlike mice, humans exposed to Candida develop immune responses early in life, so primate models may be better models for human infections. Delivered into Active vaccines for body in a virus Well tolerated vaginitis caused by particle. There are still challenges yet to overcome associated with the high cost and risks of developing a vaccine. Gaining the technical skills requires to manufacture, store and transfer live vaccines is required. Further exploitation into gaining data in humans will promote this exciting research area. Vaccination is the most effective and economical way to protect the organism against infectious diseases. Most infectious agents enter the body through the mucous membranes of the digestive, respiratory and urogenital systems. To analyze the prospects of the use of edible vaccines in the prevention of infectious diseases. The advantages of mucosal protection include: improved efficiency, simpler administration of the drug, reducing the risk of contamination by other microorganisms compared to injection or other methods that violate the skin. However, mucosal protective physiological mechanisms have removed any surface antigens from their own, including the participation of enzymes. Traditionally, this is done using the packaging - biodegradable polymeric or lipid particles, which are often administered orally or intranasally. Another more modern approach is to obtain transgenic plants which produce protective antigenic proteins of infectious agents, and their use as edible vaccines. Plant cell walls effectively protect the antigen present in them after entering the human oral cavity, swallowing, and subsequent passage through the stomach. Other attractive properties include biological safety (in which there are no viral and other human and animal pathogens), ease of storage and use.
Falciani C generic 10 mg altace otc arrhythmia statistics, Lozzi L buy discount altace 2.5mg on line arrhythmia test, Pini A, Corti F, Fabbrini M, Bernini A, Lelli B, Niccolai N, Bracci L. Synthetic peptides in the form of dendrimers become resistant to protease activity. Infuence of selective fuorination on the biological activity and proteolytic stability of glucagon-like peptide-1. Blood-to-central nervous system entry and stability of biphalin, a unique double-enkephalin analog, and its halogenated derivatives. Benedetti F, Berti F, Budal S, Campaner P, Dinon F, Tossi A, Agrirova R, Gen- ova P, Atanassov V, Hinkov A. Kobayashi K, Oishi S, Hayashi R, Tomita K, Kubo T, Tanahara N, Ohno H, Yoshikawa Y, Furuya T, Hoshimo M, Fujii N. Synthesis and Evaluation of Silanediols as Highly Selective Uncompetitive Inhibitors of Human Neutrophil Elastase. Effcient Routes to Carbon-Silicon Bond Forma- tion for the Synthesis of Silicon-Containing Peptides and Azasilaheterocycles. Kinetic decon- jugation: gateway to the synthesis of Xxx-Gly (E)-alkene dipeptide isosteres. Wangsell F, Gustafsson K, Kvarnstrom I, Borkakoti N, Edlund M, Jansson K, Lindberg J, Hallberg A, Rosenquist A, Samuelsson B. Copper mediated defuorinative allylic alkylation of difuorohomoallyl alchohol deriva- tives directed to an effcient synthetic method for (Z)-fuoroalkane dipeptide isosteres. Diastereoselective syn- thesis of the Leu-Pro type phosphinyl dipeptide isosteres tetrahedron asymmetr. That is, the chapter emphasized on improving the pharmacological activity, that is, potency of peptide drugs. We urge the unfamiliar readers to read our disclaimers and about peptide nomenclature in Sections 5. In this chapter, we will concentrate our discussion on enhancing the pharmacokinetic properties of peptide drugs with an emphasis on membrane permeability. To enhance the oral bioavailability of an active lead drug, one must realize that oral bioavailability involves several factors, such as gastrointestinal transit and absorption, chemical stability in the gastrointestinal tract, and the frst-pass effect of gut wall and liver metabolism. Lipinski formulated a rule of thumb to evaluate if a drug has properties that would make it a likely orally active drug in humans [1]. The rule states that, in general, an orally bioavailable drug should have no more than one violation of the following criteria. Peptide drugs are generally perceived as large molecules and would have diffculty crossing membranes. Most researchers correlate molecular size with molecular weight, and have set out the general rule of thumb that orally bioavailable drugs should be less than 500 g/mol. This description has been further refned by others to orally bioavailable drugs with a molecular weight between 160 to 480 g/mol [2]. As we have described in Chapter 5 we noticed that most orally bioavailable peptide drugs are comprised of three to fve residues that fts into three to fve subsites of the active site. An aspect of our work on β-secretase inhibitors and Alzheimer’s disease will be used to illustrate methods of reducing the molecular size of a peptide design. The subse- quent aggregation of these peptide amyloid β-peptide fragments leads to the pathol- ogy of the disease. In the frst method, we synthesized compounds in which one amino acid was systematically removed at a time from the N-terminal, then from the C-terminal. A nearly complete loss of inhibitory activity on the removal of a residue indicated that the position of the residue was important for active site recognition. Because glycine does not have a side-chain, any near loss of β-secretase inhibition suggested that the interactions between the side-chain of the residue and its associated subsite were important at the affected position. The resulting pentapeptide was optimized at the two end-terminals Universal Free E-Book Store... A wonderful discovery of a potent non-peptide inhibitor of β-secretase by another research group [6] inspired us to shift our focus on non-peptides.
Each of the in- in a fabricated food discount 5mg altace otc blood pressure medication hair loss, the phrase "for gredients used in the food shall be de- manufacturing" may be omitted from clared on the label as required by the any declaration of ingredients required applicable sections of parts 101 and 130 under §101 discount altace 10mg with amex heart attack 6 hours. Cocoa is the food that by intimately mixing and grinding conforms to the definition and stand- chocolate liquor with one or more op- ard of identity, and is subject to the re- tional nutritive carbohydrate sweet- quirements for label declaration of in- eners, and may contain one or more of gredients for breakfast cocoa in the other optional ingredients specified §163. The name of the tracting from the weight of the choco- food is "cocoa" or "medium fat cocoa". The finished sweet chocolate con- lll", the blank being filled in with tains less than 12 percent by weight of the common or usual name of the spe- total milk solids based on those dairy cific alkali ingredient used in the food. The following "Spice added", "Flavored with lll", safe and suitable ingredients may be or "With lll added", the blank being used: filled in with the common or usual (1) Cacao fat; name of the spice, flavoring, or sea- (2) Nutritive carbohydrate sweet- soning used, in accordance with §101. Each of the in- condensed skim milk, nonfat dry milk; gredients used in the food shall be de- (iv) Concentrated buttermilk, dried clared on the label as required by the buttermilk; and applicable sections of parts 101 and 130 (v) Malted milk; or of this chapter. The name of the by intimately mixing and grinding food is "sweet chocolate", "sweet choc- cacao fat with one or more of the op- olate coating", "semisweet chocolate", tional dairy ingredients specified in "semisweet chocolate coating", "bit- paragraph (b)(2) of this section and one tersweet chocolate", or "bittersweet or more optional nutritive carbo- chocolate coating", as appropriate. I (4–1–10 Edition) one or more of the other optional in- "With lll added", the blank being gredients specified in paragraph (b) of filled in with the common or usual this section. White chocolate shall be name of the spice, flavoring, or sea- free of coloring material. Each of the in- as calculated by subtracting from the gredients used in the food shall be de- weight of the total fat the weight of clared on the label as required by the the milkfat, dividing the result by the applicable sections of parts 101 and 130 weight of the finished white chocolate, of this chapter. The following one or more of the other optional in- safe and suitable ingredients may be gredients specified in paragraph (b) of used: this section. The finished (v) Malted milk; milk chocolate contains not less than (3) Emulsifying agents, used singly or 3. The following of chocolate, milk, or butter; safe and suitable ingredients may be (5) Antioxidants; and used: (6) Whey or whey products, the total (1) Cacao fat; amount of which does not exceed 5 per- (2) Nutritive carbohydrate sweet- cent by weight. The name of the (3) Spices, natural and artificial food is "white chocolate" or "white flavorings, ground whole nut meats, chocolate coating. Each of the in- (ii) Milk, concentrated milk, evapo- gredients used in the food shall be de- rated milk, sweetened condensed milk, clared on the label as required by the dried milk; and applicable sections of parts 101 and 130 (iii) Skim milk, concentrated skim of this chapter. Buttermilk chocolate (5) Emulsifying agents, used singly or is the food that conforms to the stand- in combination, the total amount of ard of identity, and is subject to the re- which does not exceed 1. The name of the food is "buttermilk chocolate", "but- or "Processed with lll", the blank being filled in with the common or termilk chocolate coating", "sweet buttermilk chocolate", "sweet butter- usual name of the specific neutralizing agent used in the food. Skim milk chocolate "Spice added", "Flavored with lll", is the food that conforms to the stand- or "With lll added", the blank being ard of identity, and is subject to the re- filled in with the common or usual quirements for label declaration of in- name of the spice, flavoring, or sea- gredients for milk chocolate in soning used, in accordance with §101. I (4–1–10 Edition) added beyond that amount that is nor- ments for label declaration of ingredi- mally present in the specified dairy in- ents for sweet chocolate in §163. The name of the (1) In the preparation of the product, food is "skim milk chocolate", "skim cocoa or a mixture of cocoa and choco- milk chocolate coating", "sweet skim late liquor is used in such quantity milk chocolate", or "sweet skim milk that the finished food contains not less chocolate coating". Mixed dairy product specified in paragraph (b) of this sec- chocolates are the foods that conform tion are used; and to the standard of identity, and are (3) The requirement in §163. The fats, oils, and stearins (iii) Any dairy ingredients specified may be hydrogenated; in §163. The name of the referred to in paragraph (a)(1) of this food is "sweet cocoa and vegetable fat section, exclusive of any added sweet- coating". Alternatively, the common ener or other dairy-derived ingredient or usual name of the vegetable derived that is added beyond that amount that fat ingredient may be used in the name is normally present in the specified of the food, e. The name of the conforms to the definition and stand- food is "chocolate", or "chocolate ard of identity, and is subject to the re- coating", preceded by the designation quirements for label declaration of in- of the type of milk ingredients used as gredients for sweet chocolate in prescribed in paragraph (a) of this sec- §163. Sweet cocoa and vege- cluding only those dairy ingredients re- table fat coating is the food that con- ferred to in §163. The lll oil coating", the blank being fats, oils, and stearins may be hydro- filled in with the common or usual genated; name of the specific vegetable fat used. The name of the Subpart A [Reserved] food is "sweet chocolate and vegetable Subpart B—Requirements for Specific fat coating". Alternatively, the com- Standardized Tree Nut and Peanut mon or usual name of the vegetable de- Products rived fat ingredient may be used in the name of the food, e.
By far the more common problem 2.5mg altace with amex blood pressure chart pregnant, however buy altace 10mg line arrhythmia khan academy, is medicine that simply does 3 Some regulatory authorities may accept standards below those in international phar- macopeias. In such cases, a drug that would be generally regarded as substandard might be technically acceptable in a given country. The supporting text describes the committee’s understanding of a substandard drug. Poor-quality medicines cause treatment failure, but doctors do not generally suspect medicines as a cause of disease progression. Lifesaving medicines can be of poor quality, which may be an uncounted root cause of high mortality in low- and middle-income countries. Medications for chronic and infectious diseases alike have been found falsifed and sub- standard. A considerable body of research indicates that inexpensive anti- microbial drugs in low- and middle-income countries are frequently of poor quality. Such drugs not only put patients at risk but also encourage drug resistance, thereby threatening population health for future generations. Substandard antimicrobials often contain low and erratic drug doses, while falsifed ones can be diluted. In either case, exposing pathogens to subtherapeutic doses of medicines selectively allows the growth of resistant organisms. Drug-resistant staphylococcus infections are an emerging problem, especially in India, Latin America, and sub-Saharan Africa. An- timalarial resistance threatens to undo the good that artemisinin therapies have done, threatening global malarial control programs. Medicines are expensive; patients and governments waste money on ineffective ones. Lingering illnesses decrease productivity, causing work- ers to forgo pay and spend more on treatment. Through encouraging antimicrobial resistance, illegitimate medicines reduce the effective life of a drug. Society must bear the cost of drug development, an expense that increases as drugs become more complex. Substandard and falsifed medicines undermine confdence in the health system and in all public institutions. Their sale fnances other crimes, buys weapons and ammu- nition, and conveys power to corrupt offcials. Victims of falsifed and sub- standard drugs usually do not even know they are victims and are therefore deprived of their right to redress. In many ways, the trade in illegitimate pharmaceuticals further erodes the already weak political infrastructure that allows them to circulate, part of a vicious cycle of poverty and crime. Governments and industry monitor problems with drug qual- ity, but this information is not usually public. The Pharmaceutical Security Institute, a network of the security divisions of 25 major pharmaceutical companies, has data that indicate that the illegal trade and manufacture of medicines is a global problem. It affected at least 124 countries in 2011, and the burden is disproportionately felt in the developing world. Interpol, an international organization that facilitates police cooperation, has conducted 11 opera- tions against illicit medicines since 2008. Police working in Interpol raids have confscated tons of suspect products, leading to hundreds of investiga- tions and arrests. Much of the scientifc literature about drug quality is in case studies: reports from clinicians who uncover substandard or falsifed drugs in their routine work. This kind of report provides context on how and when dif- ferent kinds of drugs are compromised; it can also trigger epidemiological investigation. Nonprobability or convenience samples are by far the most commonly used method to study drug quality.