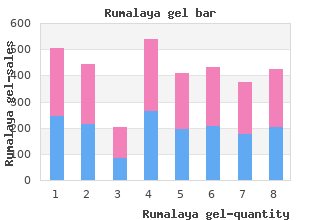
Rumalaya gel
By F. Esiel. Alcorn State University.
Information regarding a drug’s use in pediatrics is usually not included in the drug’s package insert purchase rumalaya gel 30gr visa spasms during sleep. Instead generic rumalaya gel 30gr online muscle relaxant suppository, statements similar to the following appear: “not approved for use in children younger than 12 years of age” or “safety and efficacy in children has not been established. Reporting systems must be improved and reporting needs to be mandatory instead of voluntary. With this knowledge, it is hoped that testing can be performed before therapy to avoid treatment tragedies. Medication errors in children: a descriptive summary of medication error reports submitted to the United States Pharmacopeia. The impact of hospital wide com- puterized physician order entry on medical errors in a pediatric hospital. Survey of adverse drug reactions on a pediatric ward: a strategy for early and detailed defection. Topical creams and ointments were limited to topical rather than systemic effects. Dosage forms became more advanced during the 1950s and 1960s; however, drug delivery technology was mainly limited to sustained-release delivery via the oral route. An example of an oral sustained-release formulation from this period is the Spansule capsule technology developed by Smith Kline and French Laboratories. As the pellets travel down the gastrointestinal tract, the coating material dissolves to release the drug. By using a capsule containing pellets incorporating a spectrum of different thickness coatings (and thus dissolution rates), sustained drug release of a given pattern is possible. It was not until the 1970s, with the advent of dedicated drug delivery research companies, that significant advances in drug delivery technology were made. The recognition that specific research had to be undertaken in order to overcome the problems of conventional drug delivery led to the evolution of modern- day pharmaceutical science and technology. The phenomenal advances in the fields of biotechnology and molecular biology gave an additional impetus to drug delivery research in the 1980s and early 1990s. These advances provided large quantities of new biopharmaceuticals, such as peptides, proteins and antisense oligonucleotides, which generally possess inherent disadvantages for drug delivery. Disadvantages include such properties as large molecular size, hydrophilicity and instability, making these “new biotherapeutics” unsuitable for oral delivery. Generally such drugs must be given by the parenteral route, which has many associated disadvantages, as mentioned above. Recent research has been directed towards the use of alternatives to the parenteral route, for drugs (including the “new biotherapeutics”) that cannot be delivered orally. Potential alternative portals of drug entry to the systemic circulation include the buccal, sublingual, nasal, pulmonary and vaginal routes. These routes are also being studied for the local delivery of drugs directly to the site of action, thereby reducing the dose needed to produce a pharmacological effect and also possibly minimizing systemic side-effects. Drug delivery technology is becoming increasingly sophisticated and current approaches take into account such factors as the influence of pharmacokinetic processes on drug efficacy, as well as the importance of drug timing and of drug targeting to the site of action. Emerging technologies are addressing a variety of issues, including bio-responsive drug release and the delivery of nucleic acid therapeutic entities. This book is concerned with the various routes of delivery under investigation, and these new and 3 emerging delivery technologies. However, a full appreciation of these concerns cannot be gained without first understanding: • the concept of bioavailability; • the process of drug absorption; • the pharmacokinetic processes; • the importance of timing for optimal drug therapy; • delivery considerations for the “new biotherapeutics”; • the limitations of conventional therapy. This chapter provides an overview of these considerations and highlights the necessity for advanced drug delivery systems, in order to optimize drug efficacy. In terms of drug efficacy, the bioavailability of a drug is almost as important as the potency of the active agent itself. Measuring a drug’s bioavailability thus involves measuring the rate and extent of drug absorption. This is ideally measured in terms of the clinical response of a patient; however, only a minority of clinical responses, such as blood pressure, can provide accurate quantitative data for analysis. A further method of assessment is the measurement of the drug concentration at the site of action; however, this cannot be achieved practically. For clinical purposes, it is generally accepted that a dynamic equilibrium exists between the concentration of drug at the site of action (C ) and the concentration of drug in blood plasma (C ).
In the case of leishmaniasis 30gr rumalaya gel sale muscle relaxant cream, the most recent example is the registration in India of the anticancer miltefosine as an antileishmanial drug cheap rumalaya gel 30gr without prescription muscle relaxant tea. Indeed, several anticancer drugs have shown activity against both cancer and Leishmania (Miguel et al. The high-throughput screening of compound libraries using whole parasite is gaining new relevance in Plasmodium, Trypanosome and Leishmania spp. Among the screened libraries of compounds are the ones made of natural products (reviewed in Tagboto et al. The target-based drug discovery is a very expensive and time consuming rational approach, but it permits increased knowledge of parasite biology. The selection of a target based on genomics screening implies its validation by genetic or chemical approaches. Moreover, the target should be biochemical and structurally characterized, subject to selective inhibition without developing resistances, and technically accessible to the screening of several compounds (Pink et al. Different laboratories have engineered genetically defined mutant Leishmania parasites, aimed at identifying parasite virulence or disease persistence factors that could allow the identification of either potential drug targets or attenuated live vaccines. The progresses in the characterization of some of the above-listed proteins’ potential as drug targets and the search for inhibitors are mentioned on the review paper included in this dissertation. The therapies available up to the present are far from satisfactory and, since leishmaniasis affects poor people in poor regions, the development of new drugs has been ne- glected due to the lack of commercial motivation. Safe and orally available drugs, especially against the visceral form of the disease, are needed. An overview of the main strategies for antileishmanial drug development, mainly focused on the target-based drug development approach, is given. Given that the available therapy is far maniasis, is responsible for serious mankind diseases in from satisfactory, there are, according to the World Health tropical and subtropical areas of the world. Amphotericin the inoculation of infective flagellated promastigotes that B revealed itself to be active not only against fungal infec- invade or are phagocytosed by local or recruited host cells. The amphotericin B deoxycholate (Fungizone®) into non flagellated amastigotes that multiply and are able to is a second line treatment, except in Bihar State in India, infect other adjacent or distant macrophages. This drug’s main drawback is is dependent on drug therapy, since no approved vaccine is that it has the potential to induce acute toxicity requiring available. It has many advantages, including the Rua do Campo Alegre, 823, 4150-180 Porto, Portugal; Tel: 0035122 short course of treatment, oral efficiency and low cost. Drugs for the Treatment of Leishmaniasis covered following several approaches, including the combi- nation of available commercial drugs, the discovery of new Current Therapy Sodium Stibogluconate (Pentostam) applications for existing drugs and the discovery of new Meglumine antimoniate (Glucantime) Amphotericin B (Fungizone) molecules. The latter could be achieved by screening com- Liposomal Amphotericin B (Ambisome) pound libraries using whole parasites or, instead, using a Miltefosine (India and Colombia) previously validated parasite target. From among the strate- gies mentioned, we will focus on the target-based drug dis- Clinical Trials Paromomycin covery. This rational approach is both very expensive and Imiquimod time consuming, but it permits the increase of knowledge of Sitamaquine parasite biology. The selection of a target based on genomics screen- active in vitro against Leishmania, just a few have been ing implies its validation by genetic or chemical approaches. Conse- Moreover, the target should be biochemical and structurally quently, it is easy to summarize the number of molecules, characterized, subject to selective inhibition without devel- which, in clinical trials, are active against leishmaniasis. Be- oping resistances, and technically accessible to the screening longing to the aminoglycoside antibiotic family, paromomy- of several compounds [23]. Protection from viru- more common in patients receiving paromomycin than in lent challenges in mice was reached with mutant parasites patients receiving amphotericin B (6% vs. This compound is capable of stimulating a local immune response, suggesting its potential The bifunctional enzyme dihydrofolate reductase application in several situations. Even though the combi- esting target for the development of drugs against protozoa nation of imiquimod and antimoninals has produced good parasites [24]. Only a few molecules that selectively inhibit the leish- for tropical parasitic diseases, involving integrated partner- Therapy and Further Development of Anti-Leishmanial Drugs Current Drug Therapy, 2008, Vol.
Health Officers Council of British Columbia (2005) A public health approach to drug control generic 30 gr rumalaya gel with visa spasms parvon plus. The Health Officers Council of British Columbia (2011) Public health perspectives for regulating psychoactive substances: what we can do about alchohol generic rumalaya gel 30 gr otc spasms of the stomach, tobacco, and other drugs. Grover A (2010) Report of the Special Rapporteur on the Right of Everyone to the Enjoyment of the Highest Attainable Standard of Physical and Mental Health (Item 69(b) of the provisional agenda of the sixty-fiftession of the United Nations General Assembly). House of Commons Home Affairs Select Committee The government’s drugs policy: is it working? The Advisory Group on Drug and Alcohol Education (2008) Drug education: an entitlement for all a report to government by the advisory group on drug and alcohol education. Faggiano F, Vigna-Taglianti F, Versino E et al (2005) School-based prevention for illicit drugs use. Lloyd C, Joyce R, Hurry J et al (2000) The effectiveness of primary school drug education. Home Office (2009) Blueprint drugs education: the response of pupils and parents to the programme – executive summary. Joseph Rowntree Foundation (2005) Random drug testing of school children: a shot in the arm or a shot in the foot for drug prevention. Her Majesty’s Government (2010) Drug strategy 2010: reducing demand, restricting supply, building recovery: supporting people to live a drug free life. National Institute on Drug Abuse (2006) Evaluation of the national youth antidrug media campaign: 2004 report of findings. National Institute for Health and Clinical Excellence (2006) Drug use prevention among young people: a review of reviews. Department of Health (2000) Vulnerable young people and drugs: opportunities to tackle inequalities. Hammersley R, Marsland L & Reid M (2003) Substance use by young offenders: the impact of the normalisation of drug use in the early years of the 21st century. Fishbein M, Hall-Jamieson K, Zimmer E et al (2002) Avoiding the boomerang: testing the relative effectiveness of antidrug public service announcements before a national campaign. House of Commons Home Affairs Select Committee The government’s drugs policy: is it working? Jaffe J & O’Keeffe C (2003) From morphine clinics to buprenorphine; regulating opioid antagonist treatment of addiction in the United States. Haasen C, Verthein U & Degkwitz P (2007) Heroin-assisted treatment for opioid dependence: randomised controlled trial. National Institute for Health and Clinical Excellence (2007) Methadone and buprenorphine for the management of opioid dependence. Her Majesty’s Government (2010) Drug strategy 2010: reducing demand, restricting supply, building recovery: supporting people to live a drug free life. Robins L (1993) Vietnam veterans rapid recovery from heroin addiction: a fluke, or normal expectation? Recovery Orientated Drug Treatment Group, National Treatment Agency for Substance Misuse (2012) Medications in recovery. Hubbard R, Marsden M, Rachel J et al (1989) Drug abuse treatment: a national study of effectiveness. Bell J, Dru A, Fischer B et al (2002) Substitution therapy for heroin addiction Substance Use and Misuse 37: 1145-74. Romelsjö A, Engdahl B, Stenbacka M et al (2010) Were the changes to Sweden’s maintenance treatment policy 2000-06 related to changes in opiate-related mortality and morbidity? De Maeyer J, Vanderplasschen W & Broekaert E (2010) Quality of life among opiate-dependent individuals: a review of the literature. Moffatt S, Weatherburn D & Donnelly N (2005) What caused the recent drop in property crime? Rosenbaum M (1985) A matter of style: variation among methadone clinics in the control of clients.
Evaluation of World Health Organization criteria for antiretroviral treatment failure in resource-limited settings purchase rumalaya gel 30gr without prescription muscle relaxant injections. Evaluating patients for second-line antiretroviral therapy in India: the role of targeted viral load testing cheap rumalaya gel 30gr spasms hiccups. Failure of immunologic criteria to appropriately identify antiretroviral treatment failure in Uganda. The role of targeted viral load testing in diagnosing virological failure in children on antiretroviral therapy with immunological failure. Dried blood spots perform well in viral load monitoring of patients who receive antiretroviral treatment in rural Tanzania. Guidelines for the psychosocially assisted pharmacological treatment of opioid dependence. Effect of concomitantly administered rifampin on the pharmacokinetics and safety of atazanavir administered twice daily. Effect of rifampin on steady-state pharmacokinetics of atazanavir with ritonavir in healthy volunteers. Pharmacokinetics of adjusted-dose lopinavir-ritonavir combined with rifampin in healthy volunteers. Resistance in pediatric patients experiencing virologic failure with first- and second-line antiretroviral therapy. Treatment outcomes of patients on second-line antiretroviral therapy in resource-limited settings: a systematic review and meta-analysis. Cardiovascular risk factors in adult Malawians on long-term antiretroviral therapy. Transactions of the Royal Society of Tropical Medicine and Hygiene, 2011, 105:644–649. Length/ height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age. Adherence to medication regimens among children with human immunodeficiency virus infection. Adherence to antiretroviral therapy during and after pregnancy in low-income, middle-income, and high- income countries: a systematic review and meta-analysis. Depression, alcohol use and adherence to antiretroviral therapy in sub-saharan Africa: a systematic review. Interventions to increase antiretroviral adherence in sub-Saharan Africa: a systematic review of evaluation studies. Distribution of antiretroviral treatment through self-forming groups of patients in Tete Province, Mozambique. Ambassadors for adherence: provision of highly effective defaulter tracing and re-engagement by peer educators in Tanzania. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. A pilot study of food supplementation to improve adherence to antiretroviral therapy among food-insecure adults in Lusaka, Zambia. Challenges in using mobile phones for collection of antiretroviral therapy adherence data in a resource- limited setting. Supporting patient adherence to antiretrovirals using mobile phone reminders: patient responses from South India. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Mobile phone technologies improve adherence to antiretroviral treatment in a resource-limited setting: a randomized controlled trial of text message reminders. Medication diaries do not improve outcomes with highly active antiretroviral therapy in Kenyan children: a randomized clinical trial. Adult patients’ adherence to anti-retroviral treatment: a survey correlating pharmacy refill records and pill counts with immunological and virological indices. Pharmacy adherence measures to assess adherence to antiretroviral therapy: review of the literature and implications for treatment monitoring. Validation of self-report and hospital pill count using unannounced home pill count as methods for determination of adherence to antiretroviral therapy.
Recreational use Many people are able to use psychoactive substances in a recreational manner (see Glossary) that causes no problems to the individual or those around them generic rumalaya gel 30 gr free shipping muscle relaxants sleep. This pattern of use is usually characterised by moderate levels of consumption and periods when the person stops using the substance without difficulty order rumalaya gel 30 gr mastercard spasms right side abdomen. Harmful, dependent and hazardous use There are clear, internationally agreed frameworks for describing harmful and dependent patterns of substance use. These frameworks define a hierarchy of physical, psychological and social harm to the individual. Within the chapter on mental and behavioural disorders, a subchapter defines mental and behavioural disorders due to psychoactive substance use. It defines a number of categories including acute intoxication (see Glossary), harmful use, dependence and withdrawal. The level of harm caused by a particular pattern of substance use is defined by the categories ‘harmful’ and ‘dependent’. Psychological dependence involves a need (craving – see Glossary) for repeated doses of the drug to feel good, or avoid feeling bad. Physiological (physical) dependence is associated with tolerance (see Glossary), where increased doses of the drug are required to produce the effects originally produced by lower doses, and development of withdrawal syndrome (see Glossary) when the drug is withdrawn. Withdrawal syndrome is characterised by physiological and psychological symptoms that are specific to a particular drug. The term ‘dependence’ is often used interchangeably with ‘addiction’ (see Glossary). In contrast to harmful use, hazardous use also refers to patterns of use that are of public health significance, despite the absence of any current disorder in the individual user. These terms, and many others that are used throughout the report, are discussed in more detail in the Glossary. Substances have been clearly shown to affect the brain in the short and longer term. Some substances (eg heroin, cannabis) mimic endogenous neurotransmitters, while others (eg cocaine, amphetamine) increase the availability of endogenous neurotransmitter to the brain, by either increasing neurotransmitter release or inhibiting its breakdown. If a person uses substances over a longer period of time, the brain’s structure and function begin to change, prompting behavioural changes in that individual. The prefrontal cortex area of the brain is particularly vulnerable to the effect of substances. This brain area is crucial for decision making, such as weighing up the pros and cons of a certain activity. Research suggests that the prefrontal cortex is one of the last brain areas to mature. It is a naturally occurring, ‘feel good’ neurotransmitter that is important in rewarding positive behaviours (eg eating, drinking). Some psychoactive substances cause dopamine to be released rapidly and in huge quantities when compared to usual brain levels. Raised levels of dopamine in the mesolimbic system lead to intense feelings of pleasure, known to users as a ‘high’ (see Glossary). If substance use persists, the brain responds to the dopamine overstimulation by decreasing the amount of dopamine produced and reducing the number of dopamine receptors (see Glossary) available. This, in turn, can lead to the user feeling emotionally flat and exhausted once the immediate effect of the drug has subsided. The user will often try to stimulate further additional dopamine release by using larger quantities of the substance. The role of dopamine in the effect of psychoactive drugs is considered further in Section 4. Genetics There is strong evidence for a genetic component to dependence, provided by family, twin and adoption studies (see Chapter 4). Although research suggests many genes may be involved,18 there is evidence that a single genetic variant in the aldehyde dehydrogenase 2 gene impacts on patterns of drinking and the risk of dependence. The genetics of dependence is a rapidly developing area but, apart from the studies on the aldehyde dehydrogenase 2 gene, there is little immediate prospect of a breakthrough in genetics leading to improved patient care. As described above, dependence can be considered primarily a brain disorder, but one that interacts with a range of predisposing, precipitating, perpetuating and protective factors. These factors can best be described in a framework in which the biological, psychological and social components are identified. Psychological factors include comorbid mental health problems such as depression, psychosis and personality disorder.
Information systems can be paper-based or based on an electronic registry proven rumalaya gel 30gr spasms on right side of stomach, depending on local context buy rumalaya gel 30gr low price muscle relaxant pregnancy safe. Programmes should develop a systematic strategy for collecting and aggregating key information that supports better management of the patient and ensures high-quality care. A robust patient information system is also critical for high-quality monitoring and evaluation of programmes and for supply management systems. When effective operational solutions such as successful service delivery models and processes of care are identifed in existing systems, programmes need to consider scaling up such models of care. Issues to be considered include mobilizing and allocating resources; training, mentoring and supervising health workers; procuring and managing drugs and other medical supplies; and monitoring and evaluation. In most generalized epidemic settings, maternal and child health services are provided at the primary care level, where pregnant women and children predominantly access health services. The quality of some of these studies was downgraded because of relatively few events (65–70). All these factors increased the satisfaction of the people receiving care and may have contributed to improving the quality of care (66,71). Guidance on operations and service delivery 189 and another showed comparable mortality rates. The quality of evidence was weighed along with programmatic risks and benefits; acceptability; values; preferences; cost implications; feasibility; critical contextual constraints; and contextual relevance. Plans for provider-initiated testing and counselling in such settings should emphasize supportive social, policy and legal frameworks (64). Rationale and supporting evidence In many countries, people who inject drugs are a marginalized population with limited access to and utilization of health care services. Randomized trials found that opioid substitution therapy decreases illicit drug use and increases retention in care relative to placebo (98). Observational studies found that opioid substitution therapy decreases mortality relative to not being in care (100). Some studies observed trends for improved viral suppression and reduced mortality, whereas others found comparable rates of viral suppression and mortality (101–103). In several settings, transport cost is a significant barrier to access and retention in care. Attrition declined after 12 months, resulting largely from significantly reduced losses to follow-up. All health workers, including community health workers, need to be regularly trained, mentored and supervised to ensure high-quality care and the implementation of updated national recommendations. The use of new technologies such as computer-based self-learning, distance education, online courses and phone-based consultation may supplement classroom in-service training and support the effcient use of health workers’ time and other resources (116,117). Although volunteers can make a valuable contribution on a short-term or part- time basis, all trained health workers who are providing essential health services, including community health workers, should receive adequate wages and/or other appropriate and commensurate incentives (116). Task shifting involves the rational redistribution of tasks among health workforce teams. With this approach, specifc tasks are reassigned, where appropriate, from highly qualifed health workers to health workers with shorter training and fewer complementary qualifcations to more effciently and effectively use the available human resources. Task shifting should be implemented alongside other strategies designed to increase the total numbers and capacity of all types of health workers. Rationale and supporting evidence The systematic review identifed three randomized trials and six observational studies addressing task shifting. The quality of care in these studies was ensured by (1) providing training, mentoring, supervision and support for nurses, non-physician clinicians and community health workers; (2) ensuring clear indications for patient referral; (3) implementing referral systems and (4) implementing monitoring and evaluation systems. Patient education could help people and their families understand that care provided by nurses and community health workers is not of lower quality than that provided by physicians (106–108,111,113,114,119–121). To ensure that testing services are accurate and reliable, relevant quality assurance systems need to be developed and strengthened. Since an increasing number of new diagnostic tests and point-of-care systems is entering the market, the use of only high-quality diagnostics and equipment needs to be ensured. Strategic planning for properly placing and harmonizing testing platforms should be carried out to ensure appropriate use and cost–effectiveness. Providing for and strengthening a dedicated, effcient, safe and cost-effective specimen referral system requires reliable specimen transport with adequate conditions for whole blood, plasma and dried blood spot specimens and rapidly and dependably reporting test results back to the referring site with linkage to care.
Ammonium discount rumalaya gel 30 gr with mastercard muscle relaxant clonazepam, flavorings order rumalaya gel 30 gr mastercard spasms ms, or seasonings specified in potassium, or sodium bicarbonate, car- paragraphs (b)(4) and (b)(5) of this sec- bonate, or hydroxide, or magnesium tion are used in the chocolate liquor, carbonate or oxide, used as such, or in the label shall bear an appropriate aqueous solution; statement, e. Phosphoric vored with lll", "Seasoned with acid, citric acid and L-tartaric acid, lll", or "With lll added", the used as such, or in aqueous solution; blank being filled in with the common (3) Spices, natural and artificial or usual name of the spice, flavoring, flavorings, and other seasonings that or seasoning used, in accordance with do not either singly or in combination §101. The name of the bined in a manner that is appropriate, food is "breakfast cocoa", or "high fat but not misleading. Lowfat cocoa is the (2) When any optional neutralizing food that conforms to the definition agent specified in paragraph (b)(2) of and standard of identity, and is subject this section is used, including those to the requirements for label declara- used in the preparation of the cacao tion of ingredients for breakfast cocoa nibs from which the breakfast cocoa in §163. Cocoa with dioctyl so- paragraph (b)(3) of this section are used dium sulfosuccinate for manufacturing in the breakfast cocoa, the label shall is the food additive complying with the provisions prescribed in §172. It conforms to the definition "Spice added", "Flavored with lll", and standard of identity, and is subject or "With lll added", the blank being to the requirements for label declara- filled in with the common or usual tion of ingredients, for breakfast cocoa name of the spice, flavoring, or sea- in §163. The name of the pears on the label so conspicuously as food additive is "cocoa with dioctyl so- to be easily seen under customary con- dium sulfosuccinate for manufac- ditions of purchase, the statements turing" to which is added any modifier prescribed in this paragraph showing of the word "cocoa" required by the optional ingredients used shall precede definition and standard of identity to or follow the name without intervening which the food additive otherwise con- printed or graphic matter. Each of the in- in a fabricated food, the phrase "for gredients used in the food shall be de- manufacturing" may be omitted from clared on the label as required by the any declaration of ingredients required applicable sections of parts 101 and 130 under §101. Cocoa is the food that by intimately mixing and grinding conforms to the definition and stand- chocolate liquor with one or more op- ard of identity, and is subject to the re- tional nutritive carbohydrate sweet- quirements for label declaration of in- eners, and may contain one or more of gredients for breakfast cocoa in the other optional ingredients specified §163. The name of the tracting from the weight of the choco- food is "cocoa" or "medium fat cocoa". The finished sweet chocolate con- lll", the blank being filled in with tains less than 12 percent by weight of the common or usual name of the spe- total milk solids based on those dairy cific alkali ingredient used in the food. The following "Spice added", "Flavored with lll", safe and suitable ingredients may be or "With lll added", the blank being used: filled in with the common or usual (1) Cacao fat; name of the spice, flavoring, or sea- (2) Nutritive carbohydrate sweet- soning used, in accordance with §101. Each of the in- condensed skim milk, nonfat dry milk; gredients used in the food shall be de- (iv) Concentrated buttermilk, dried clared on the label as required by the buttermilk; and applicable sections of parts 101 and 130 (v) Malted milk; or of this chapter. The name of the by intimately mixing and grinding food is "sweet chocolate", "sweet choc- cacao fat with one or more of the op- olate coating", "semisweet chocolate", tional dairy ingredients specified in "semisweet chocolate coating", "bit- paragraph (b)(2) of this section and one tersweet chocolate", or "bittersweet or more optional nutritive carbo- chocolate coating", as appropriate. I (4–1–10 Edition) one or more of the other optional in- "With lll added", the blank being gredients specified in paragraph (b) of filled in with the common or usual this section. White chocolate shall be name of the spice, flavoring, or sea- free of coloring material. Each of the in- as calculated by subtracting from the gredients used in the food shall be de- weight of the total fat the weight of clared on the label as required by the the milkfat, dividing the result by the applicable sections of parts 101 and 130 weight of the finished white chocolate, of this chapter. The following one or more of the other optional in- safe and suitable ingredients may be gredients specified in paragraph (b) of used: this section. The finished (v) Malted milk; milk chocolate contains not less than (3) Emulsifying agents, used singly or 3. The following of chocolate, milk, or butter; safe and suitable ingredients may be (5) Antioxidants; and used: (6) Whey or whey products, the total (1) Cacao fat; amount of which does not exceed 5 per- (2) Nutritive carbohydrate sweet- cent by weight. The name of the (3) Spices, natural and artificial food is "white chocolate" or "white flavorings, ground whole nut meats, chocolate coating. Each of the in- (ii) Milk, concentrated milk, evapo- gredients used in the food shall be de- rated milk, sweetened condensed milk, clared on the label as required by the dried milk; and applicable sections of parts 101 and 130 (iii) Skim milk, concentrated skim of this chapter. Buttermilk chocolate (5) Emulsifying agents, used singly or is the food that conforms to the stand- in combination, the total amount of ard of identity, and is subject to the re- which does not exceed 1. The name of the food is "buttermilk chocolate", "but- or "Processed with lll", the blank being filled in with the common or termilk chocolate coating", "sweet buttermilk chocolate", "sweet butter- usual name of the specific neutralizing agent used in the food. Skim milk chocolate "Spice added", "Flavored with lll", is the food that conforms to the stand- or "With lll added", the blank being ard of identity, and is subject to the re- filled in with the common or usual quirements for label declaration of in- name of the spice, flavoring, or sea- gredients for milk chocolate in soning used, in accordance with §101. I (4–1–10 Edition) added beyond that amount that is nor- ments for label declaration of ingredi- mally present in the specified dairy in- ents for sweet chocolate in §163. The name of the (1) In the preparation of the product, food is "skim milk chocolate", "skim cocoa or a mixture of cocoa and choco- milk chocolate coating", "sweet skim late liquor is used in such quantity milk chocolate", or "sweet skim milk that the finished food contains not less chocolate coating". Mixed dairy product specified in paragraph (b) of this sec- chocolates are the foods that conform tion are used; and to the standard of identity, and are (3) The requirement in §163. The fats, oils, and stearins (iii) Any dairy ingredients specified may be hydrogenated; in §163. The name of the referred to in paragraph (a)(1) of this food is "sweet cocoa and vegetable fat section, exclusive of any added sweet- coating".