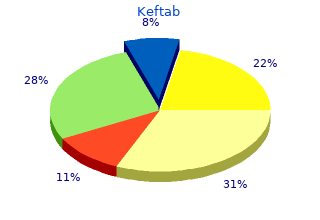
Keftab
2018, Meredith College, Gembak's review: "Keftab 750 mg, 500 mg, 375 mg, 250 mg, 125 mg. Only $0,48 per pill. Best online Keftab.".
Implementation considerations especially relevant to programme managers are provided for major new recommendations 500 mg keftab amex virus in the heart. A concluding chapter on monitoring and evaluation provides preliminary guidance on monitoring the implementation of new recommendations buy keftab 250 mg mastercard bacteria urinalysis. Modelling, expert consultations and country case studies have informed clinical, operational and programmatic guidance. The process has identified key gaps in knowledge that will guide the future research agenda. The guidelines are anticipated to guide country policy decisions and planning the scaling up of ArT. They will also be a valuable resource for clinicians and informing the priorities of development agencies, international organizations, nongovernmental organizations and other implementing partners during the next few years. Where the recommendations remain unchanged from 2010 ArT guidelines, this is clearly stated in the table. Women meeting treatment eligibility criteria should continue lifelong ArT (strong recommendation, moderate-quality evidence). In some countries, for women who are not eligible for ArT for their own health, consideration can be given to stopping the ArV regimen after the period of mother-to-child transmission risk has ceased (conditional recommendation, low-quality evidence). Breastfeeding should then only stop once a nutritionally adequate and safe diet without breast-milk can be provided (strong recommendation, high- quality evidence for the frst 6 months; low-quality evidence for the recommendation of 12 months). Countries should discontinue d4T use in frst-line regimens because of its well-recognized metabolic toxicities (strong recommendation, moderate-quality evidence). Patients on a failing second-line regimen with no new ArV options should continue with a tolerated regimen (conditional recommendation, very low-quality evidence). Special considerations Strategies that balance the benefts and risks for children need to for children be explored when second-line treatment fails. Children on a second-line regimen that is failing with no new ArV drug options should continue with a tolerated regimen. If ArT is stopped, opportunistic infections still need to be prevented, symptoms relieved and pain managed. Decentralization of The following options should be considered for decentralization treatment and care of ArT initiation and maintenance. Initiation of ArT in hospitals with maintenance of ArT in peripheral health facilities (strong recommendation, low-quality evidence). Initiation and maintenance of ArT in peripheral health facilities (strong recommendation, low-quality evidence). Initiation of ArT at peripheral health facilities with maintenance at the community level (that is, outside health facilities in settings such as outreach sites, health posts, home-based services or community-based organizations) between regular clinical visits (strong recommendation, moderate-quality evidence). Summary of new recommendations 35 operations and service delivery (continued) Topic Recommendations Task-shifting Trained non-physician clinicians, midwives and nurses can initiate first-line ArT (strong recommendation, moderate-quality evidence). Trained non-physician clinicians, midwives and nurses can maintain ArT (strong recommendation, moderate-quality evidence). Trained and supervised community health workers can dispense ArT between regular clinical visits (strong recommendation, moderate-quality evidence). Guidance for programme managers Topic Guidance Guidance for For deciding on the implementation of the clinical and programme managers operational recommendations, it is recommended that: The national authorities do so using a transparent, open and informed process. This process should have broad stakeholder engagement, including meaningful participation from the affected communities, and take into account the specifics of the recommendations under discussion. The latter would identify which inputs and systems are currently available and which areas require additional investment. The decision-making process take into account the ethics, equity and human rights, the impact and cost-effectiveness and the opportunity and risk dimensions of alternative implementation options. The 2006 updates of the guidelines (3–5) introduced the concept of a public health approach, with simplifed and harmonized ArV regimens (6). These publications and their updates, most recently in 2010 (7–9), have provided important guidance to countries that have scaled up national ArV programmes during the past decade. The ArV regimens now available, even in the poorest countries, are safer, simpler, more effcacious and more affordable than ever before. Although countries are at different stages of ArT coverage and implementation of the 2010 guidelines (7–9) and there are still important gaps in research, there is a consistent global trend towards expanding access and the earlier initiation of treatment.
Government Work Against Pharmaceutical Fraud Catherine Hill-Herndon keftab 500mg line antibiotic resistance upec, Director purchase keftab 750mg amex antibiotic 200 mg, Offce of International Health and Biodefense, U. Department of Justice Jeffery Gren, Director, Offce of Health and Consumer Goods, U. Manager of the Campaign for Access to Essential Medicines, Doctors Without Borders Ann Marie Kimball, Moderator Copyright © National Academy of Sciences. Committee members discussed the report and potential conclusions and recommendations. Goyal, Additional Secretary and Director General, Central Government Scheme, Ministry of Health and Family Welfare Arun Panda, Joint Secretary, Ministry of Health and Family Welfare 12:30-1:30 Lunch Copyright © National Academy of Sciences. Appaji, Director General, Pharmexcil India Meghana Inamdar, General Counsel and Managing Consultant, Sidvim Lifesciences Copyright © National Academy of Sciences. Countering the Problem of Falsified and Substandard Drugs Copyright © National Academy of Sciences. Structure function analysis of Leishmania sirtuin: an ensemble of in silico and biochemical studies. Characterization of the anti-Leishmania effect induced by cisplatin, an anticancer drug. The synthesis and the in vitro cytotoxicity studies of bisnaphthalimidopropyl polyamine derivatives against colon cancer cells and parasite Leishmania infantum. Differential effects of polyamine derivative compounds against Leishmania infantum promastigotes and axenic amastigotes. A Leishmania infantum cytosolic tryparedoxin activates B cells to secrete interleukin-10 and specific immunoglobulin. Leishmania cytosolic silent information regulatory protein 2 deacetylase induces murine B-cell differentiation and in vivo production of specific antibodies. Flurazepam inhibits the P-glycoprotein transport function: an insight to revert multidrug-resistance phenotype. Evaluation of the immune response following a short oral vaccination schedule with hepatitis B antigen encapsulated into alginate-coated chitosan nanoparticles. The Faculty of Pharmacy of the University of Porto (Portugal), the Institute for Molecular and Cell Biology of the University of Porto (Portugal) and the Institut de Recherche pour le Développement, Montpellier (France) provided the facilities and logistical supports. Anabela, thank you for the opportunity to give my first steps in the research under your supervision, when I was still a university th student in the 4 year of the course. Thanks also, for your guidance, demand, encouragement, trust and of course, your unconditional support during all of these years. To Dr Ali Ouaissi, I would like to thank you for the advices, guidance and support. Thank you also for the interesting discussions and for the opportunity to work with you. I would like to thank the former head of the Biochemistry department of the Faculty Pharmacy of Porto University, Prof. Fernando Sena Esteves, not only for the facilities provided to perform my work during these years, but also for his support. Of course, to all members of the Parasite Disease group, with whose, I have shared physic and intellectual space, thanks for your friendship and motivation. A special thank for all the help and support (by seniority reasons…), goes to Marta Silva, Ricardo Silvestre, Nuno Santarem and Sofia Lima. Salette Reis of the Physic Chemistry department, and also to all the members of Toxicology department of the Faculty Pharmacy of Porto University for their prompt contribution in providing the facilities and/or the means in several situations of my research, without which it would be impossible to perform some experiments. To Madalena Pinto (Madazinha…), Lucilia Saraiva, Helena Castro and Helena Vasconcelos thank you so much for your friendship, support and motivation. I thank also all the members of the Biochemistry department of the Faculty Pharmacy of Porto University. To my dear Alexandra Ferreira I acknowledge her valuable help in the English revision of this dissertation (Is it correct?
Instances of a darkened urine have also been reported discount keftab 125mg with mastercard antibiotics dosage, and this manifestation has been the subject of a special investigation generic keftab 750mg mastercard hpv virus. Although the pigment which is probably responsible for this phenomenon has not been positively identified, it is almost certainly a metabolite of metronidazole and seems to have no clinical significance. Infusion (ventilated): Dilute 3mg/kg in 50ml 5% dextrose and run at 0-5ml/hr (0-5mcg/ kg/min) Intranasal: Sedation: 0. The following paradoxical reactions have been observed: Excitability, irritability, aggressive behavior, agitation, nervousness, hostility, anxiety, sleep disturbances, nightmares and vivid dreams. Hepatic: Hepatomegaly, transient elevations of serum transaminases and alkaline phosphatase. Patients with renal impairment on milrinone infusions may develop progressive vasodilation leading to escalating noradrenaline requirements. If noradrenaline requirement is increasing consider whether it is appropriate to cease milrinone. Significant hypotension due to peripheral vasodilation is common and is generally treated with noradrenaline. Milrinone may aggravate outflow tract obstruction in hypertrophic subaortic stenosis. Respiratory depression occurs most frequently in the elderly and debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia when even moderate therapeutic doses may dangerously decrease pulmonary ventilation. Morphine should be used with extreme caution in patients with chronic obstructive pulmonary disease or cor pulmonale, and in patients having a substantially decreased respiratory reserve, hypoxia, hypercapnia, or preexisting respiratory depression. In such patients, even usual therapeutic doses of morphine may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Hypotensive Effect Morphine sulphate controlled-release tablets, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain his blood pressure has already been compromised by a depleted blood volume, or a concurrent administration of drugs that lower blood pressure. Respiratory: Respiratory depression, apnoea, respiratory arrest, Gastrointestinal: Dry mouth, biliary tract spasm, laryngospasm, anorexia, diarrhoea, cramps, taste alteration, constipation, ileus, intestinal obstruction, increases in hepatic enzymes. Cardiovascular: Flushing of the face, chills, tachycardia, bradycardia, palpitation, faintness, syncope, hypotension, hypertension. If stored at cool temperatures precipitation may occur – this will redissolve at room temperature. Moxifloxacin, given as an oral tablet, is well absorbed from the gastrointestinal tract. Aerobic Gram-Positive Microorganisms: Staphylococcus aureus (methicillin-susceptible strains only), Streptococcus pneumoniae (including penicillin-resistant strains), Streptococcus pyogenes. Aerobic Gram-Negative Microorganisms: Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella pneumoniae, Moraxella catarrhalis. Convulsions and neuropsychiatric complications Convulsions have been reported in patients receiving quinolones. Hypersensitivity Reactions Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving quinolone therapy. Pseudomembranous Colitis Pseudomembranous colitis has been reported with nearly all antibacterial agents, including moxifloxacin, and may range in severity from mild to life-threatening. Peripheral Neuropathy Rare cases of sensory or sensorimotor axonal polyneuropathy affecting small and/or large axons resulting in paraesthesias, hypoesthesias, dysesthesias and weakness have been reported in patients receiving quinolones. Tendon Effects Ruptures of the shoulder, hand, achilles tendon or other tendons that required surgical repair or resulted in prolonged disability have been reported in patients receiving quinolones. Oral administration of quinolones with antacids containing aluminum or magnesium, with sucralfate, with metal cations such as iron, or with multivitamins containing iron or zinc, or with formulations containing divalent and trivalent cations such as (didanosine) chewable/buffered tablets or the paediatric powder for oral solution, may substantially interfere with the absorption of quinolones, resulting in systemic concentrations considerably lower than desired. Central Nervous System: Insomnia, nervousness, anxiety, confusion, somnolence, tremor, vertigo, paraesthesia. Naloxone prevents or reverses the effects of opioids including respiratory depression, sedation and hypotension. In such cases, an abrupt and complete reversal of narcotic effects may precipitate an acute abstinence syndrome. Several instances of hypotension, hypertension, ventricular tachycardia and fibrillation, and pulmonary edema have been reported.
Atropine may be given but may not be required because of the rapidly reversible type of cholineste- rase inhibiton produced (oximes should not be given) buy keftab 125mg low cost antibiotics z pack dosage. Iron Poisoning and Iron and Aluminium Overload: Mortality from iron poisoning is reduced by specifc therapy with desferrioxamine which chelates iron buy keftab 500mg without a prescription antibiotic treatment for mrsa. Before administra- ton of desferrioxamine the stomach should be empted by gastric lavage (with a wide-bore tube) within 1 h of ingestng a signifcant quantty of iron or if radiography reveals tablets in the stomach. It is used in the diagnosis of aluminium overload and to treat aluminium overload in patents with end- stage renal failure undergoing maintenance haemodialysis. Heavy Metal Poisoning: Heavy metal poisoning may be treated with a range of ant- dotes including dimercaprol, penicillamine, potassium ferric hexacyanoferrate and Sodium calcium edetate. Methaemoglobinaemia: Methylthioninium chloride can lower the levels of methae- moglobin in red blood cells and is used in the treatment of methaemoglobinaemia. In large doses, it may cause methae- moglobinaemia and therefore methaemoglobin levels should be monitored during treatment. Cyanide Poisoning: Cyanide poisoning may be treated with Sodium nitrite followed by Sodium thiosulphate. Following that infusion of atropine at 10-20 % of total inital dose required/hour; may require boluses during infusion. Contraindicatons In myasthenia gravis (but may be used to decrease muscarinic side-efects of antcholinesterases), paralytc ileus, pyloric stenosis and prostatc enlargement; refux oesophagits; unstable cardiac rhythm. Precautons Elderly, Down syndrome; angle-closure glaucoma; myasthenia gravis; prostatc enlargement; pyrexia; lactaton (Appendix 7b); interactons (Appendix 6a); pregnancy (Appendix 7c). Adverse Efects Constpaton, transient bradycardia (followed by tachycardia, palpitaton and arrhythmias), reduced bronchial secretons, urinary urgency and retenton, dilataton of the pupils with loss of accommodaton, photophobia, dry mouth, fushing and dryness of the skin. Occasionally, confusion (partcularly in the elderly), nausea, vomitng and giddiness; very rarely, angle-closure glaucoma may occur. Desferrioxamine Mesylate* Pregnancy Category-C Indicatons Acute iron poisoning; chronic iron overload; aluminium overload; primary hemochromatosis. Afer 1 to 2 h reduce to 3-4 mg/kg/h for the next 22-23 hrs (max dose is 100 mg/kg over 24 hrs). Precautons Renal impairment; eye and ear examinatons before and at 3-month intervals during treatment; aluminium encephalopathy (may exacerbate neurological dysfuncton); children under 3 years (may retard growth); lactaton; interactons (Appendix 6c). Adverse Efects Anaphylaxis; fushing, urtcaria, hypotension, shock (especially if given by too rapid intravenous infusion); gastrointestnal disturbances; fever, headache, arthralgia, myalgia; arrhythmias; renal impairment; blood disorders; neurological disturbances including neuropathy, paraesthesia and dizziness; convulsions; Yersinia and mucormycosis infectons; visual disturbances (including lens opacity and retnopathy) and hearing loss; rash; rarely, growth retardaton (in young children); rarely, acute respiratory distress syndrome; pain on intramuscular or subcutaneous injecton; local irritaton on prolonged subcutaneous infusion; reddish- brown discolouraton of urine. Precautons Hypertension; renal impairment (discontnue or use with extreme cauton if renal failure occurs during treatment); any abnormal reacton such as hyperpyrexia should be assessed; elderly; pregnancy (Appendix 7c); lactaton, alkalinize urine to pH of 7. Adverse Efects Hypertension, tachycardia; malaise, nausea, vomitng, abdominal pain, salivaton, lacrimaton, sweatng, burning sensaton in the mouth, throat and eyes; feeling of constricton in throat and chest; headache, muscle spasms, tngling of the extremites; fever in children; local pain and abscess at injecton site, iron toxicity potentaton. Child- 20 mg/kg/day administered in 3-4 divided doses, initatng treatment at 25% of this dose and gradually increasing to full dose over 2-3 weeks to minimize adverse reactons. Precautons Monitor throughout treatment including blood counts and urine tests; renal impairment; immunosuppressive treatment; avoid oral iron within 2 h of a dose; hepatc impairment; pregnancy (Appendix 7c). In Wilson’s disease, consider withdrawal if platelet count falls below 120 000/mm3 or white blood cells below 2500/mm3 or if 3 successive falls within reference range (can restart at reduced dose when counts return to reference range but permanent withdrawal necessary if neutropenia or thrombocytopenia recur). In Wilson’s disease warn patent to tell doctor immediately if sore throat, fever, infecton, non-specifc illness, unexplained bleeding and bruising, purpura, mouth ulcers or rashes develop. Adverse Efects Initally nausea (less of a problem if taken with food and on retring), anorexia, fever; taste loss (mineral supplements not recommended); blood disorders including thrombocytopenia, neutropenia, agranulocytosis and aplastc anaemia; proteinuria, rarely, haematuria (withdraw immediately); haemolytc anaemia, nephrotc syndrome, lupus erythematosus- like syndrome, myasthenia gravis-like syndrome, polymyosits (rarely, with cardiac involvement), dermatomyosits, mouth ulcers, stomatts, alopecia, bronchiolits and pneumonits, pemphigus, Goodpasture syndrome and Stevens-Johnson syndrome also reported; male and female breast enlargement reported; rash early in treatment (usually allergic-may need temporary withdrawal), late rashes (reduce dose or withdraw treatment). Flumazenil* Pregnancy Category-C Indicatons Antdote for benzodiazepine overdose, reversal of sedatve efects produced by benzodiazepenes administered during general anaesthesia or diagnostc or therapeutc procedures. Contraindicatons Epilepsy, neuromuscular blockade, hypersensitvity to benzodiazepines, patents of suspected tricyclic antdepressant overdose, raised intracranial pressure. Precautons History of seizures, panic atack, alcohol drug dependence, bleeding disorder, liver disease, head injury, respiratory depression, pregnancy (Appendix 7c). Adverse efects Convulsions, fatgue, injecton site pains, increased sweatng, facial erythema, raised intracranial pressure, agitaton, dizziness, abnormal vision, may cause complete heart block, fushing, transient increase in blood pressure and heart-rate. Methylene Blue (Methylthioninium Chloride)* Pregnancy Category-C Indicatons Acute methaemoglobinaemia. Dose Intravenous injecton Methaemoglobinaemia caused by high dosage of prilocaine infusion: 1-2 mg/kg intravenously over 5 minutes, followed immediately by a fuid fush of 15-30 ml to minimize local pain. Contraindicatons Severe renal impairment; methaemoglo- binaemia due to chlorate or induced by sodium nitrite in treatment of cyanide poisoning; afects ability to drive machinery.